15. DISTILLATION FUNDAMENTALS
Module Objectives
1. Explain the differences between boiling and condensing for pure substances and
mixtures
2. Describe single stage distillation as a tool to illustrate the principles of distillation
3. Describe the relationship between temperature and composition in distillation
4. Apply the principle of a single stage distillation to achieve higher purity separation
5. Explain the purpose and function of reflux and reflux ratio
6. Distinguish between the different modes of operation for distillation
7. Summarize equilibrium in distillation
8. Demonstrate how to find composition in a phase diagram
9. Identify elements and flow for a distillation system
10. Explain the design and function of trays and other column internals
11. Describe equilibrium in relation to column trays and associated temperature,
pressure, and composition relationships and variables that affect distillation
12. Describe heat and material balance for distillation systems
13. Explain fractionation and vacuum distillation
14. List the methods used to evaluate performance of a distillation
Module Introduction
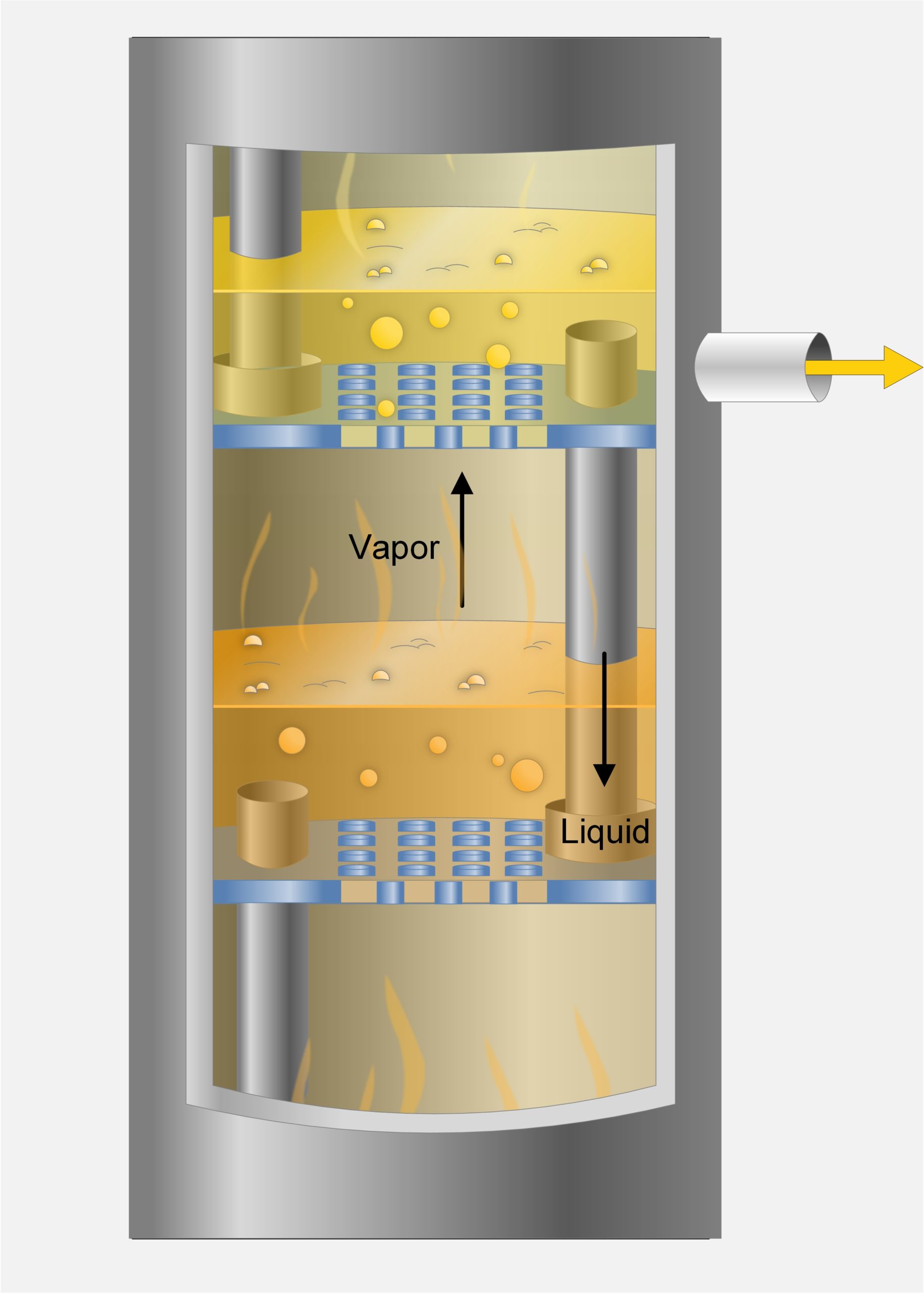
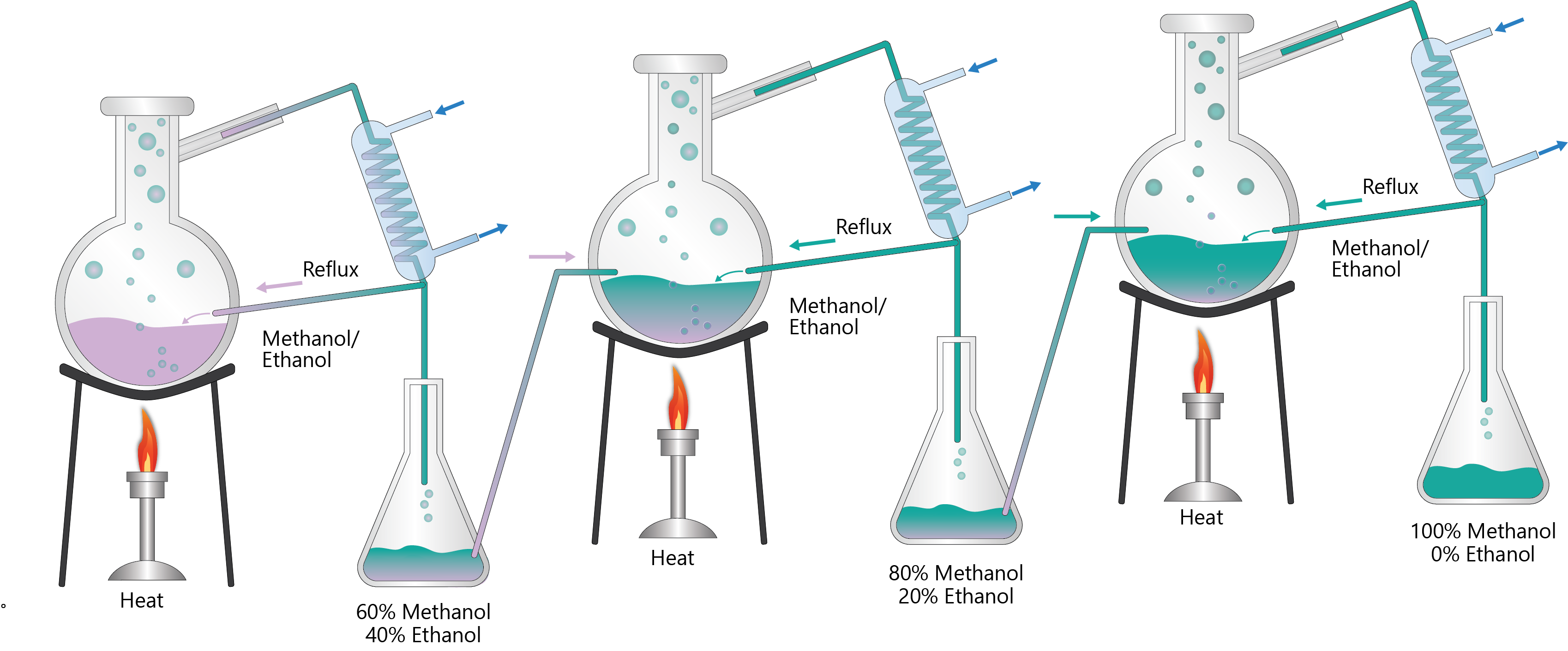
Distillation is a thermal separation process based on vaporization and condensation. It is widely used in refineries and some chemical plants. A mixture of two or more components can be separated when the boiling points of the component in a mixture are significantly different. Distillation is typically preformed in distillation columns.
[product_table id=”6020″]
$100.00